Basic Info:
General Description 1: A yellow or gray powder that has an unpleasant odor. It is used as a solid lubricant. TiS2 is very toxic for humans. If it comes in contact with water, hydrogen sulfide is produces, which is a toxic flammable gas. Inhaling the gas can cause severe injury or death.
Fire Hazard. TiS2 is moisture sensitive and is very flammable when it comes into contact with moisture or air.
Thin films of TiS2 are used in Lithium Ion Batteries 2
Belongs to the transition metal dichalcogenides 3
TiS2 nanotubes can be used as a hydrogen storage material 3
Crystal Structure:
Lattice Parameters 4:
a = 3.40 Å
c = 5.69 Å
c/a = 1.67
hexagonal, space group P3m1,
Basic Parameters at 300 K:
Band structure and carrier concentration:
Carrier Concentration: 9 x1020 cm-34
A graph showing band structure for TiS2 can be seen at reference 3
Temperature Dependencies
A graph showing the temperature dependence of resistivity can be seen at reference 3
Electrical Properties:
Room temperature conductivity 5: 7.6 x10-1 ohm-1 cm-1
A graph of electrical conductivity vs temperature can be seen at reference 4
Basic Parameters of Electrical Properties:
TiS2 is a Semimetallic 3
Mobility and Hall Effect 5:

A graph of Hall Mobility vs Temperature can be seen at reference 4
Optical properties:
A graph of the measured reflectivity spectrum of TiS2 vs calculated spectra can be seen at reference 6
Absorption coefficient vs. phonon energy, etc.
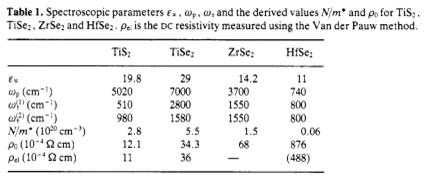


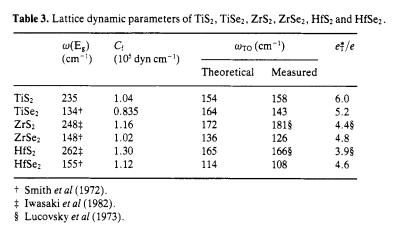
Thermal properties
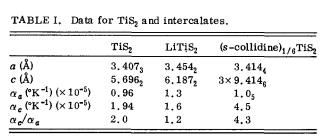
Lattice parameters with temperature coefficients 7
Developed at the University of Utah primarily by undergraduate students Jeff Provost and Carina Hahn working with Prof. Mike Scarpulla. Caitlin Arndt, Christian Robert, Katie Furse, Jash Sayani, and Liz Lund also contributed. The work was fully supported by the US National Science Foundation under the Materials World Network program award 1008302. These pages are a work in progress and we solicit input from knowledgeable parties around the world for more accurate or additional information. Contact [email protected] with such suggestions.
- 1. “TITANIUM(IV) SULFIDE CAS No. 12039-13-3”. .
- 2. “CVD routes to titanium disulfide films”, Advanced Materials, vol. 6, no. 3, pp. 237 - 239, 1994.
- 3. a. b. c. d. e. “Electronic structure of TiS2 and its electric transport properties under high pressure”, Journal of Applied Physics, vol. 109, no. 5, p. 053717, 2011.
- 4. a. b. c. d. “Electrical properties of the Group IV disulfides, titanium disulfide, zirconium disulfide, hafnium disulfide and tin disulfide”, Inorganic Chemistry, vol. 7, no. 3, pp. 459 - 463, 1968.
- 5. a. b. “Hall coefficient and reflectivity evidence that TiS 2 is a semiconductor”, Journal of Physics C: Solid State Physics, vol. 12, p. L521, 1979.
- 6. Citekey Vaterlaus1985 not found
- 7. “Intercalation and lattice expansion in titanium disulfide”, The Journal of Chemical Physics, vol. 62, no. 4, p. 1588, 1975.
